Mumbai: The Ministry of Health and Family Welfare (MoHFW) issued a gazette on November 17, 2022: GSR 823(E). Copies of the official gazette were made available to the public on June 15, 2022, where, objections and suggestions received from the public on the draft rules have been considered by the central government.
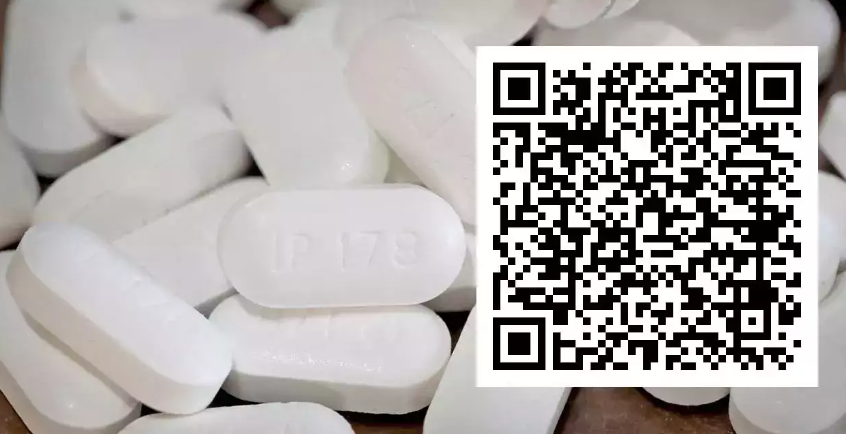
The highlight of the notification is: Manufacturers of drug formulation products as specified in Schedule H2 shall print or affix a barcode or quick response code (QR) on their primary packaging label or, in case of inadequate space in the primary package label, on the secondary package label that store data or information legible with a software application to facilitate authentication. It will become mandatory for pharmaceutical companies to affix QR codes/barcodes on the pack of 300 formulations as specified in Schedule H2 from August 1, 2023.
The QR code/barcode on being scanned shall include the following particulars: unique product identification code, proper and generic name of the drug, brand name, name and address of the manufacturer, batch number, date of manufacturing, date of expiry and manufacturing licence number. This has been implemented to curb the sales of counterfeit and spurious drugs that have been flooding the Indian market.
ETHealthworld spoke to some industry experts to understand the implementation and impact of this initiative in curbing the sales of fake drugs that the Indian pharma industry is struggling with.
Combatting menace of counterfeit drugs
SV Veeramani, Vice-Chairman, Pharmexcil is of the view that Indian pharma companies are following protocols when it comes to labelling drugs but QR codes can increase the traceability of the drugs.
He said, “QR code will be an added advantage where the patient will be assured of a genuine drug coming from a genuine company.”
Shiva Kabra, Joint MD, Control Print Limited believes that implementing QR codes is an effective method of preventing the counterfeiting of goods, and as wireless and mobile technologies advance, the process of verifying the authenticity of a drug will be simplified for the average consumer.
Adding to this, Prabir Das, Head- Packaging Tech Services, OSD (India) Mylan Laboratories Limited shared, “QR codes are touchless connectors between physical and digital information. There is no strong evidence available if a simple QR Code with static or dynamic data is used as an anti-counterfeit feature on the packaging. The data and information captured in a QR code and how that data ensures authenticity are what we need to look for to combat the menace of counterfeiting. That’s why a new variant of QR codes evolved to make it more reliable for authentication and thereby act as an anticounterfeit tool. Such QR codes are serialised or secure QR codes with few add-on features embedded in a normal QR code.”
Security measures preventing duplication of QR codes
Static QR codes can be cloned easily if they contain only data and information and do not contain any security features such as unique serial numbers, secured weblinks, password protection or an embedded image that cannot be copied easily.
Commenting on how duplication of QR codes can be addressed, Veeramani mentioned that earlier QR codes were static and the newer QR codes will be dynamic. The newer codes will have a batch number, date of manufacturing and expiry which can prevent copying.
Kabra shared how they are trying to prevent QR codes from being duplicated, he said, “The QR codes are non-fungible tokens generated on Blockchain, which can be generated only once in the system. Each code on each retail unit is variable and unique. No two codes are the same. After being used once at the point of sale (POS), the same QRs cannot be used again and their scan will warn the user about counterfeit products.
Just the implementation of dynamic QR codes on the packaging of drugs is not going to be enough to eliminate spurious drugs from the market. “Using secure QR codes can reduce the probability of reproduction of fake QR codes. But QR codes alone cannot combat the issue of fake codes or eliminate counterfeiting. Supply chain security also needs to be strengthened to ensure restricted entry of fake goods into the network through unauthorised dealers, distributors or stockists,” noted Das.
Discussing how the implementation of QR codes could benefit all that are involved, Das commented, “First and foremost requirement is awareness building of the end users to know the process of authentication through this feature. Once they are educated, it will be easier for them to find out the genuineness of the product pack. It is just like common people using QR codes for currency transactions through mobile phones in retail outlets. The benefit is that users will be instantly assured of the genuineness of the product on their hand and this will eventually help in trust-building on the brand and its manufacturer.”
Adding QR codes on a pack is not a costly affair. However, the background set-up required to make the system work requires investment. The tune of the investment may vary depending on the QR code selection and its content. “Maintaining a databank for each product pack and easy, instant access to those data and information is critical for the system to work smoothly. In a business process where multiple suppliers, multiple brands and multiple levels of transactions happen, a central server with access control will be more helpful to store only the authenticated data from all the registered users which can trigger a ‘real’ or ‘fake’ prompt on scanning the QR code. Establishing such a system requires bigger investment with collaboration from the industry and government,” concluded Das.
Some of the benefits to end users and pharma companies will be existing government compliance, GS-1 norms fulfilment, end-to-end track and trace, QC check and rejection accounting, complete business intelligence suite with short-term expiry data, real-time stock data, real-time stock transfers, distributor incentive tracking, product recall.
This implementation of QR codes/barcodes to Schedule H2 drugs may address the plague of counterfeit drugs in the Indian market but isn’t going to eradicate the sale of fake or sub-par drugs in the market. Policymakers, the pharma industry, deployment of cutting-edge technology need to be united to address the issue of counterfeiting which is a nuisance for the pharma industry in terms of revenue loss, tarnishing of brand image and it may become a public health issue if stringent measures are not implemented on time.